Polypropylene Recycling
For decades, we have been hearing about recycling, especially plastics. Education on recycling for most of us in our 40s started in elementary school, with programs, ads, etc., promoting its benefits and how you can help mitigate the effects of climate change. Unfortunately, we are now learning that most plastics are not recycled. We now know that media and corporations lied to us about the recycling programs they created, doping us into using more plastics than needed.
Polyethylene (PE) and polypropylene (PP) are the most used polymers. Over 80 million metric tons of PP were made in 2021, and less than 2% was recycled. A study published by Greenpeace found that no plastics, including soda bottles which are the most common items, meet the requirements to be called “recyclable.” Over 827,000 tons are collected annually from American households, and almost all of it is sent to landfills.
Proper recycling reduces carbon dioxide and other greenhouse gas emissions that lead to climate change. Increasing the PP recycling rate to 50% would mean taking 7.5 million cars off U.S. highways, avoiding 34,607,544 tons of CO2 emitted annually. That said, PP is a difficult polymer to recycle.
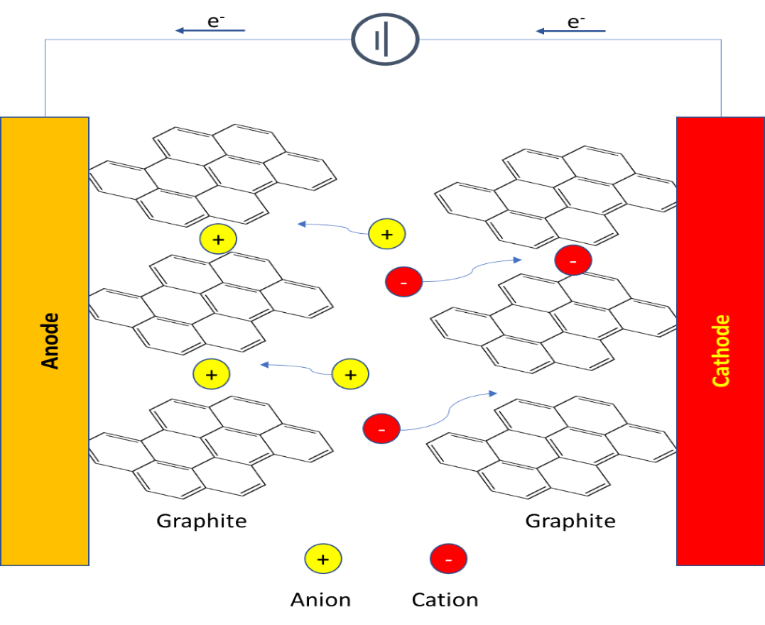
RoCo is committed to finding a solution by developing innovative technologies that will aid in the upcycling of PP. The most common containment observed in PP is PE. We are currently working on an ionic compatibilizer that is halide free, non-toxic, and miscible with polyolefins. This ionic compatibilizer overcomes phase separation. Our work is promising and has already demonstrated that a small amount of ionic compatibilizer ~0.5 wt.% significantly improves mechanical and impact properties and overcomes the stiffness-impact trade-off observed in PP contaminated with PE. The ionic nature of the compatibilizer provides an interface to form highly miscible domains resulting in overcoming phase separation.
In addition, we have observed a 30% improvement in mechanical properties in glass-fibered filled PP. If we successfully commercialize this technology, it will improve recycling rates and provide upcycled PP in the market. Based on our initial estimates, the ionic compatibilizer cost will be much lower per Kg of polymer, providing a cost-effective methodology compared to the current block copolymer approach.
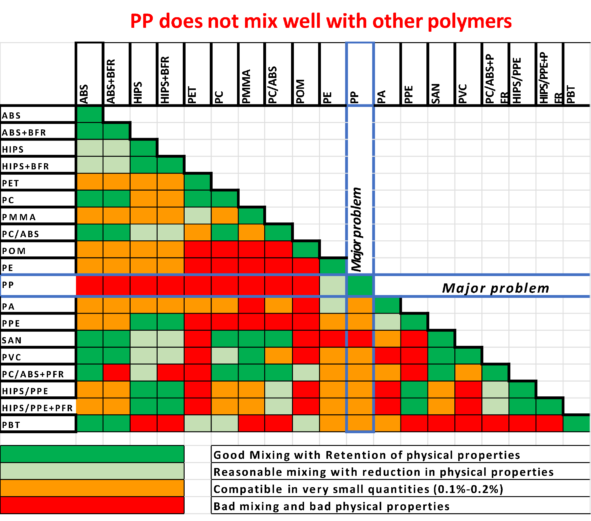
Polypropylene does not like to be mixed with other polymers. Small amounts of contaminants impact PP properties dramatically.
We are working on this goal to develop ionic compatibilizers which make PP resilient to contaminants. This results in upcycling of PP upon recycling. This would allow us to engage with industry leaders and fast-track the commercialization of this technology.
If you are interested in learning more, please contact us at info@roco.global for more information.