Ionic Liquids: Textile recycling
Due to an increase in population and demand for fast fashion, the clothing and textile industry is the 2nd largest polluter after the oil and gas industry. For instance, approximately 20,000 liters of water is needed to manufacture a T-shirt and a pair of jeans. The textile and clothing industry is globally 20% of total water waste. In addition, to water, United Nations Climate Change News reports that the clothing and fabric industry contributes to 10% of global GHG emissions. The EPA estimates that 17 million tons of textile products were generated in 2018, where only 14.7% was recycled, 19% was used in energy production, and the remaining 66.3% as landfill. According to EPA, the recycling rate has plateaued at 15% for the last 20 years(Figure 1). A major problem in minimizing textile waste is associated with consumer behavior and the in-availability of efficient technologies to reclaim, remake, and reuse. Polymer blends in textiles are a major challenge in achieving cost-effective recycling.
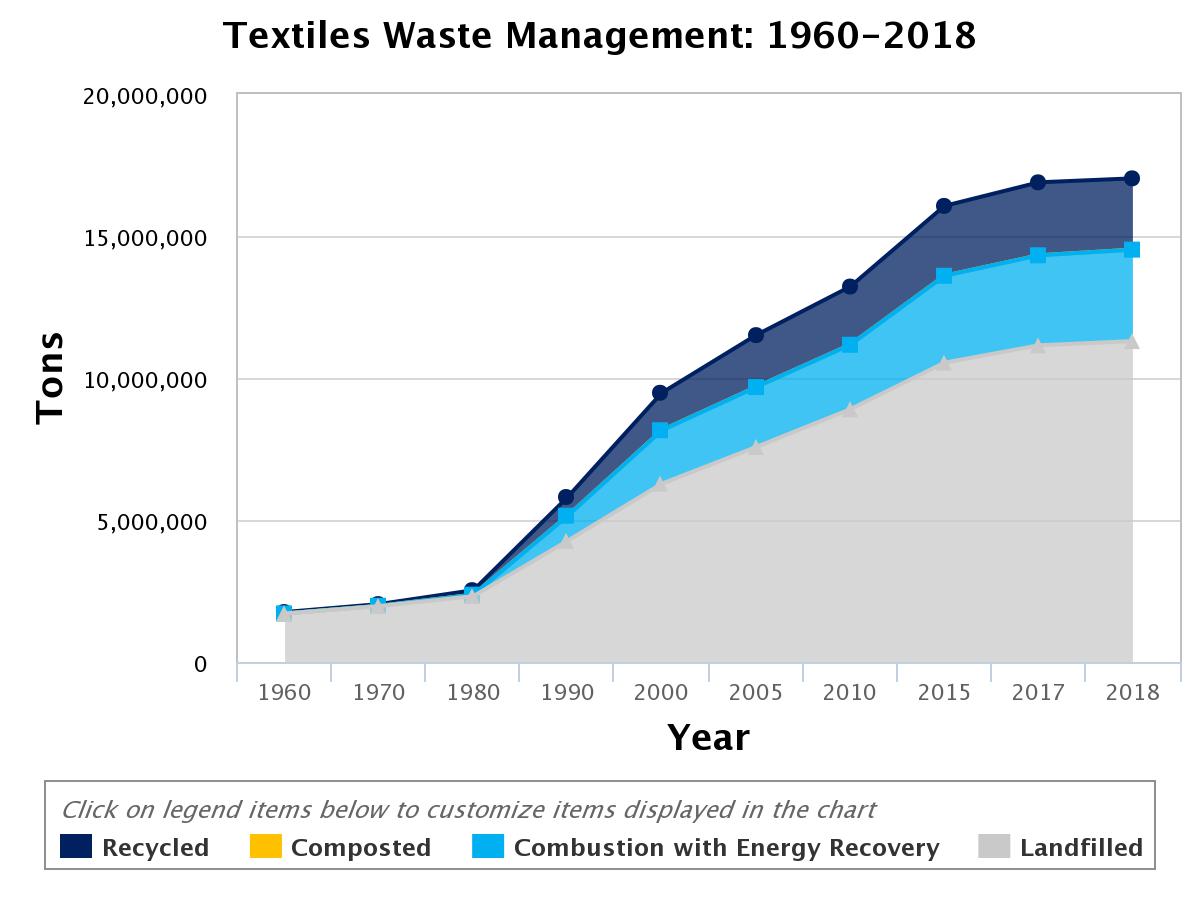
Figure 1: Textiles waste management for recycling, combustion to recover energy, and landfill
A major component in textile and fabric blends is cellulose. The traditional approaches for cellulose extraction from biomasses include the viscose and Lyocell methods. The viscose method is the most common process to extract alkali-treated cellulose using CS2. CS2 is an expensive, highly toxic, and volatile compound with known severe environmental impact. As a solvent, the lyocell process is based on N-methyl morpholine N-oxide (NMMO). It is prone to runaway reactions and solvent degradation leading to significant costs to generate fibers. These extraction technologies are unsuitable for fabric recycling due to their inefficiency and cost.
Reclaiming the polymers in fabrics is quite challenging. The blends of manmade materials and natural materials in textiles require unique approaches for separation. To create a truly circular economy, it is important to develop and deploy processes that can selectively separate cellulose from manmade fibers such as polyesters and nylons. This will result in a significant reduction in water, energy, and GHG emission.
Ionic liquids (ILs) have unique chemical properties such as low melting temperature (Tm < 100 oC), selective solubility, negligible vapor pressure, and high thermal stability (Td > 200 oC). IL-based technologies are promising for the recycling of textile waste. Due to their unique properties, ILs can decrease the amount of energy and water used. Few emerging technologies have incorporated superbase and imidazolium-based ILs as an alternative to the viscose and Lyocell processes. These ILs based technologies also show potential to convert textile wastes into high-value products. Some imidazolium-based ILs (1-ethyl-3-methylimidazolium acetate) have very high cellulose solubility (>95 grams of cellulose per mole of IL).
Sixta and coworkers at Aalto University in Finland had demonstrated the upcycling of textile wastes, including 100% cotton and cotton-polyester blends, to produce pure textile-grade cellulose. In this study, the textiles wastes were dissolved in 1,5-diazabicyclo[4.3.0]non-5-enium acetate, a superbase-based ionic liquid that selectively extracts cellulose from PET blends. The team used hydraulic pressure filtration to remove the undissolved PET fraction from the 6.5 wt% cellulose solution. The resulting solution was subjected to the dry-jet wet spinning process to make textile-grade cellulose fibers to the microfiber range (0.75 to 2.95 dtex) with breaking tenacities (27 to 48 cN/tex) and elongations (7 to 9%) comparable to commercial Lyocell fibers. This technology represents an exciting route for separating cellulose from PET enabling textile recycling.
The extracted PET cannot be used in a melt spinning to make new fibers due to the degradation of its mechanical properties. The PET must be converted into high-value materials via chemical recycling and upcycling methodologies. In the literature, numerous approaches have been investigated for the chemical recycling of PET. This includes the use of cholinium acetate (Liu et al.), cholinium phosphate (Sun et al.) and 1-butyl-3-methylimidazolium acetate (Al-Sabagh et al.). 1-butyl-3-methylimidazolium hydroxide has been used to upcycle PET (Ahmed and coworkers).
Indeed, ILs, with their unique properties, open new and environmentally friendlier ways to improve chemical recycling and upcycling of textile wastes and other synthetic polymers. Even though textile recycling is a major problem, we think it can be solved by developing innovative, cost-effective, greener solutions. RoCo is here to assist you in selecting and developing environmentally friendly technology. Are you looking for an ideal partner to help you rapidly advance your research and development initiatives on capturing and using CO2, developing high-tech functional materials, and integrating chemical recycling and upcycling using ILs? Perhaps, you want to design and test custom materials for your application? Contact RoCo Global today to learn more about our Research & Development Services and how we can help you meet, and exceed, your goals.
