Corrosion is a multi-trillion-dollar problem:
According to the National Association of Corrosion Engineers, the global cost of corrosion is estimated to be 3.4% (or $2.5 trillion) of global GDP. These costs typically exclude aspects such as individual safety or environmental consequences. Typical corrosion protection coatings are sacrificial (coating with a second metal, e.g., zinc) or barrier polymer coating, ceramic coatings, or protection oils based on paraffinic or naphthenic mineral oils.
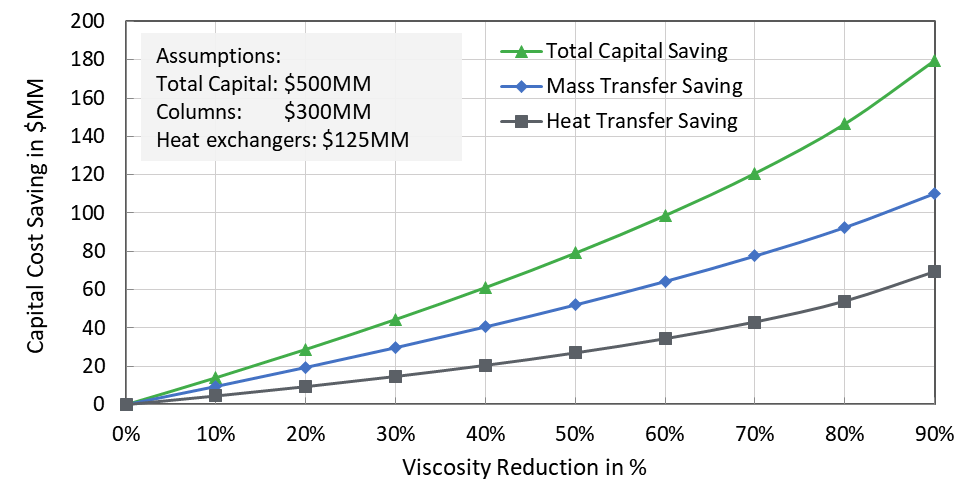
Polyoxometalates:
Polyoxometalates (POMs) are a large group of anionic polynuclear metal–oxo clusters with discrete and chemically modifiable structures. POM can exist in electron-rich reduced forms of different archetypes, structural flexibilities, and functionalities. POM materials have unique and potentially valuable catalytic, electronic, and magnetic material applications.
POM-IL as corrosion protection:
Streb and coworkers developed POM-based ionic liquids (POM-ILs), showing noticeable corrosion protection with self-healing properties. These POM-ILs are constituted by ammonium ions of the type (CnH2n+1)4N+ with n = 7-8 and transition metal (TM) functionalized Keggin anions of the type [SiW11O39TM(H2O)]n- with TM = Cu(II) or Fe(III) (Figure 1). Corrosion protection experiments were carried out on a copper disk drop-coated with the novel POM-ILs. The results were superior to coating with a solid POM salt and the commercially available IL 1-hexyl-3-methylimidazolium bromide (HMIM Br) (IL-0069-HP) as the reference.
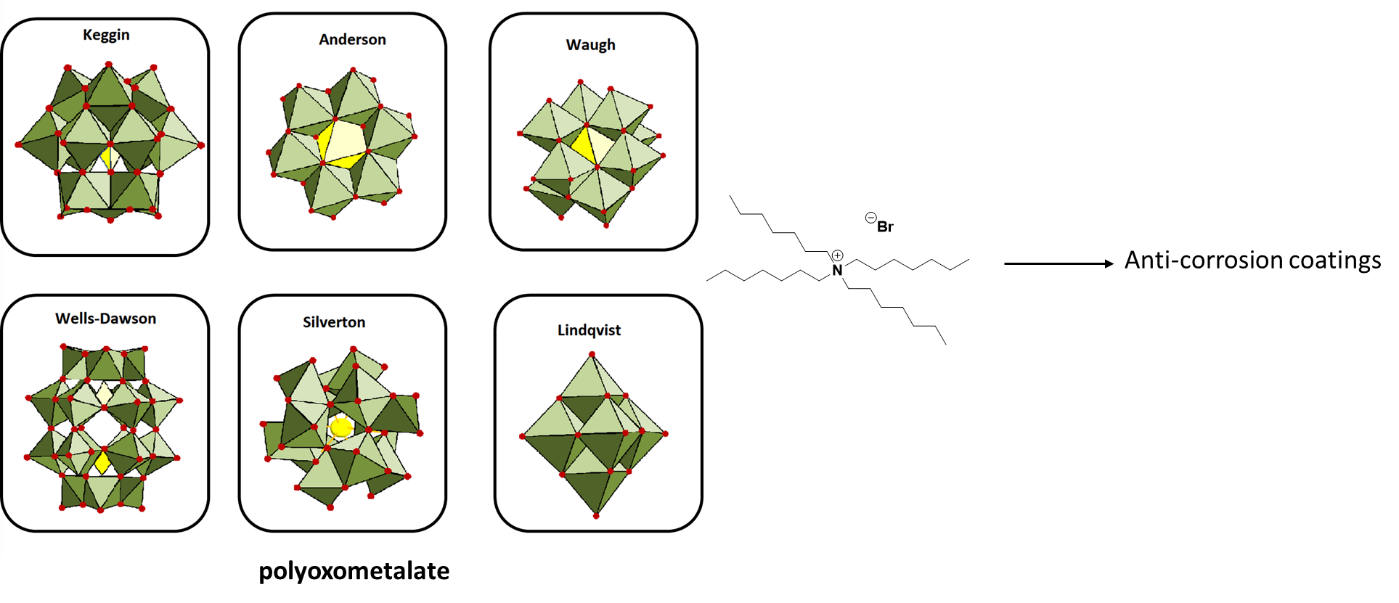
Figure 1: POM-IL resulting in anti-corrosion self-healing coating. The figure above showing mixtures of POM with ammonium-based ionic liquids.
Protection against Biodeterioration and weathering:
Another type of corrosion is the corrosion of building materials (Stones, cement, and Concrete). It is a major global issue. POM-IL coatings also show significant corrosion protection on stones and building materials as well. A 2018 study shows that the use of POM-IL thin layers can protect concrete and stone from acid corrosion (“weathering”) and biofilm formation (“biodeterioration”) (Figure 2). Stone samples are coated with hydrophobic, acid-resistant POM-ILs featuring biocidal properties that resulted in better performance.
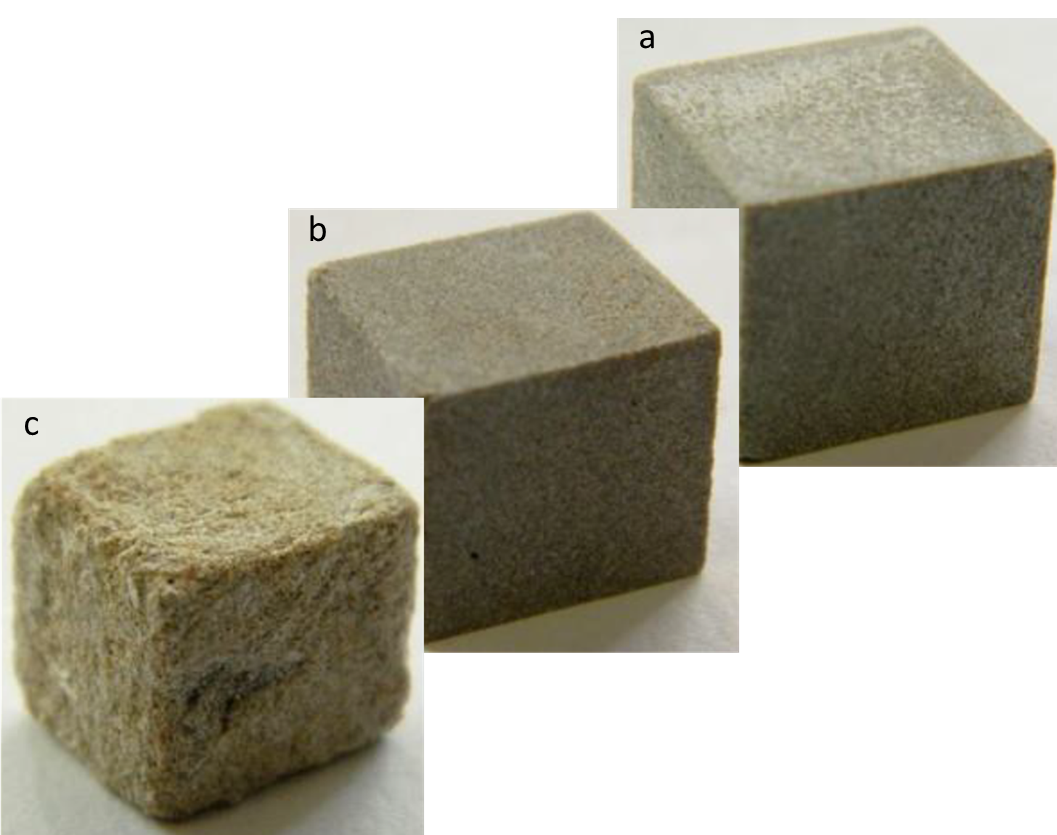
Figure 2: Images adapted from work performed by Streb 2018. The Rosemary stone was tested for acid vapor corrosion by exposing the samples to acetic acid vapor for 72 h. Sample a and b are treated with thin layer POM-ILs showing little or no acid corrosion where is C is untreated.
Our partner, IoLiTec GmBH, has tested several ionic liquids (ILs) as promising corrosion inhibitors. In this work, IoLiTec obtained optimal results with several acetate-based ILs. In addition, 1-butyl-1-methylpyrrolidinium acetate and 1- ethyl-3-methylimidazolium acetate has good corrosion protection properties. The former can be custom ordered at [email protected]. You can purchase 1-ethyl-3-methylimidazolium acetate (IL- 0189) on our website.
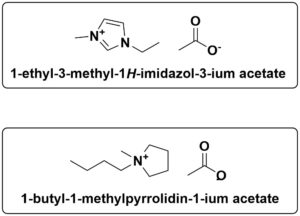 Figure 3: Chemical structure of the 1-butyl-1-methylpyrrolidinium acetate and 1- ethyl-3-methylimidazolium acetate also has good corrosion properties with POM.
Figure 3: Chemical structure of the 1-butyl-1-methylpyrrolidinium acetate and 1- ethyl-3-methylimidazolium acetate also has good corrosion properties with POM.
Besides POM-based ILs, significant anti-corrosion effects can also be obtained by subtle tuning of the IL structure. For instance, applying stable bis(trifluoromethylsulfonyl)imide (BTA) anions instead of metal invasive halides can drastically improve the anti-corrosion properties and change the cation structure. RoCo can design and synthesize novel ILs to give you an innovative edge.
Contact RoCo Global today to learn more about our Research & Development Services and how we can help you exceed your goals.