Controlling the molecular structure
Viscosity is the resistance to flow in liquids. Many factors impact viscosity such as the temperature and shape of the molecule. Temperature is quite easy to explain as higher temperatures generally corresponds to higher average kinetic energies which leads to faster-moving molecules thus lowering viscosity. There are exceptions but that would be a topic for next blog.
The molecular structure and the types of interactions impact viscosity. Within molecular interactions, hydrogen bonding plays a significant role in determining the viscosity.
The viscosity of a liquid is determined at the molecular level and it is the net results of all the interactions and the molecular weight. The VW interactions grow with molecular size. In simple molecules like oils and waxes, van der Waals (VW) forces are the key factor. Hydrocarbons are an excellent example of this behavior with a nearly linear increase in viscosity from C1 (methanol) to C10 (decanol). In more complex liquids, other factors such as the presence of double and triple bonds, molecular branching, molecular folding, ionic interactions, and hydrogen bonding are the most significant factors in determining viscosity.
Hydrogen bonding interactions are different and much stronger than VW interactions. For hydrogen bonding interactions, the number of potential bonds that can be formed between molecules is fixed but have a major impact on the viscosity. Figure 1 shows how greatly the viscosity changes as the number of hydrogen bonding goes up for three simple liquids. All three have very similar molecular weight and size, but they differ in the number of hydrogen bonds that are formed thus, resulting in huge differences in viscosity.
We have been working on understanding hydrogen bonding and how we can use it to change the viscosity of CO2 capture solvents. Figure 2 is an example where we have used computational science to gain key insights into CO2 capturing ionic liquids and by increasing the intramolecular bonding which resulted in much decreased viscosity.
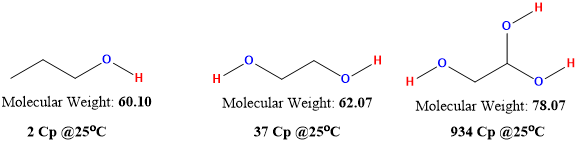
Figure 1: impact of hydrogen bonding on viscosity in simple alcohols
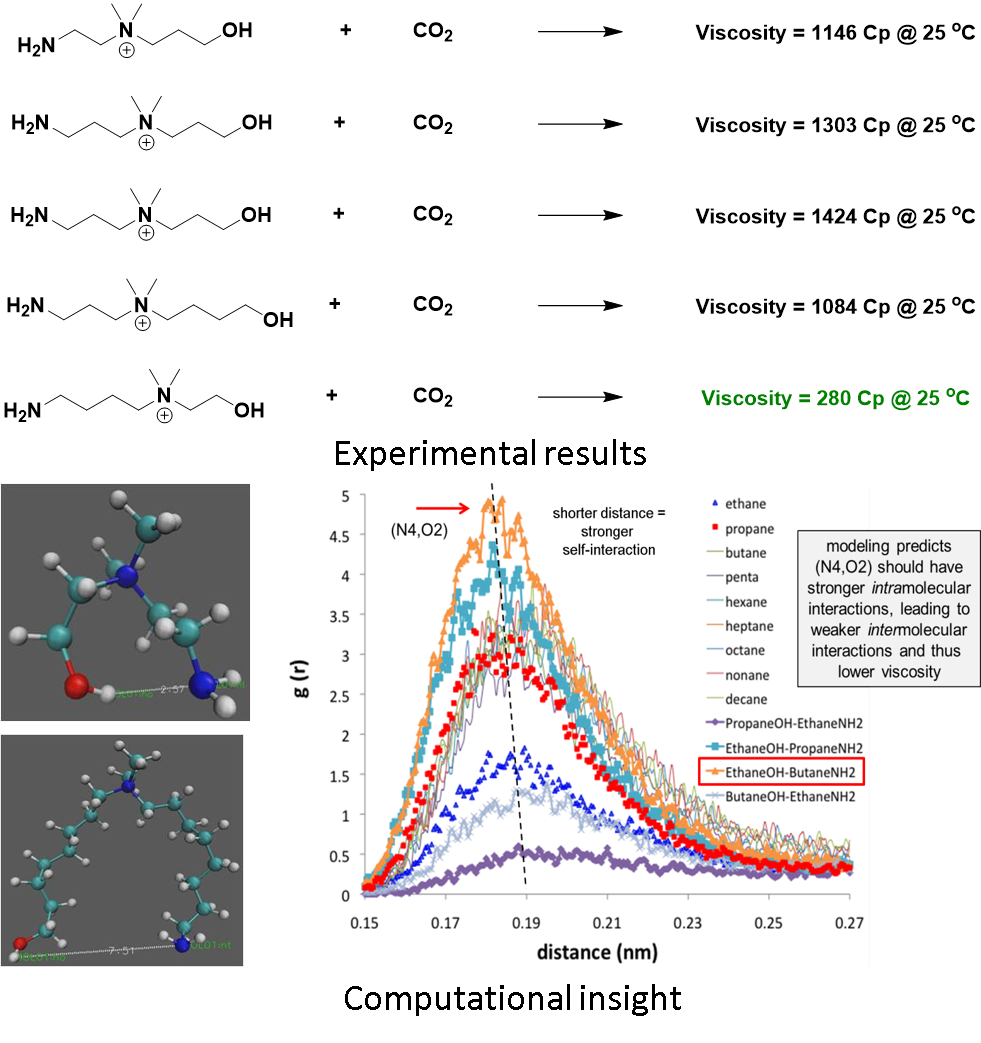
Figure 2: Molecular interactions can decrease viscosity significantly. Top: experimental results showing decrease in viscosity; Bottom: Computer simulation insights showing increase in intramolecular interactions leads to weaker intermolecular interactions and thus significantly lower viscosity.
