The need for innovative solutions in energy storage and sustainable chemistry, ionic liquids have emerged as the go to materials. One of our hottest product is 1-Methyl-1-propylpyrrolidinium bis(trifluoromethylsulfonyl)imide ([C3C1Pyrr][TFSI]), an ionic liquid that stands out for its exceptional properties and versatile applications.
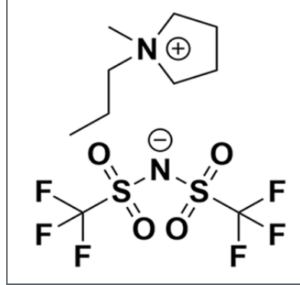 What Makes [C3C1Pyrr][TFSI] Unique?
What Makes [C3C1Pyrr][TFSI] Unique?
This ionic liquid is composed entirely of ions and exists in the liquid state at temperatures below 100°C. Its defining feature—a pyrrolidinium cation paired with a bis(trifluoromethylsulfonyl)imide anion. This particular ionic liquid has a wide electrical window, as well as excellent chemical and thermal stability. These characteristics make [C3C1Pyrr][TFSI] an ideal candidate for cutting-edge applications, including:
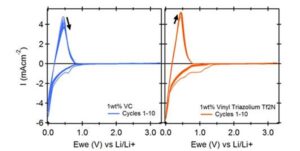
Advanced Lithium-Ion Batteries: The non-flammable nature and wide electrochemical stability window of 1-Methyl-1-propylpyrrolidinium bis(trifluoromethylsulfonyl)imide
- Lithium Ion Batteries: address critical safety concerns in current lithium-ion batteries, offering a safer alternative to traditional organic electrolytes.
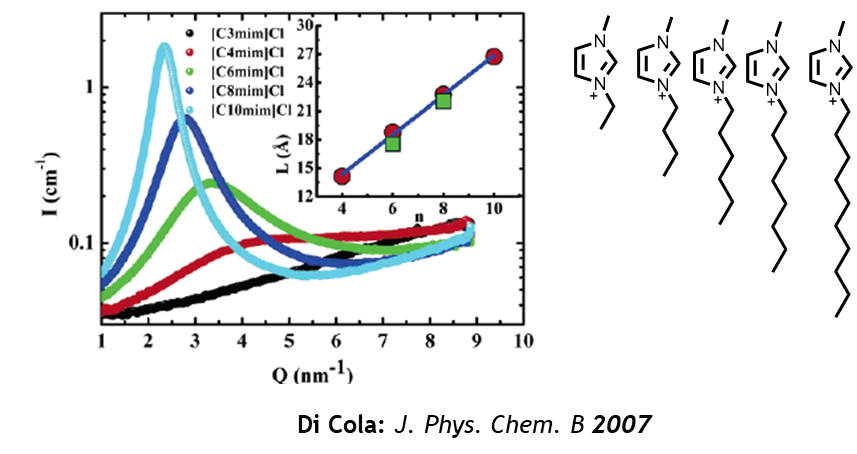
- Replacement for traditional Solvents: Its low volatility and high ionic conductivity allow [C3C1Pyrr][TFSI] to serve as an environmentally friendly solvent in chemical synthesis and material processing.
- High-Performance Capacitors: The ionic liquid’s ability to operate across a broad temperature range supports the development of energy-efficient and durable supercapacitors.
Key Benefits
- Thermal Stability: [C3C1Pyrr][TFSI] remains stable at elevated temperatures, ensuring reliability in high-demand applications.
- Non-Flammability: Its inherent safety reduces the risk of combustion, making it suitable for safer battery technologies.
- Customizable Properties: The ionic liquid can be tuned to meet specific needs by adjusting its formulation or blending with compatible materials.
Pioneering Research and Innovations
Recent studies have highlighted the potential of [C3C1Pyrr][TFSI] in energy storage. For example, research published in Batteries showcases how binary mixtures of this ionic liquid with lithium salts enhance ionic conductivity while maintaining safety and stability at varying temperatures. These breakthroughs underscore its role in paving the way for next-generation lithium batteries with superior performance metrics. (Batteries 2024, 10, 319. https://doi.org/10.3390/batteries10090319)
Summary of Research Paper
The paper titled “Pyrrolidinium-Based Ionic Liquids as Advanced Non-Aqueous Electrolytes for Safer Next Generation Lithium Batteries” provides a comprehensive analysis of [C3C1Pyrr][TFSI] and its mixtures with lithium salts. Key findings include:
- Enhanced Thermal and Electrochemical Stability: The ionic liquid offers a wide liquid range, reducing risks such as crystallization at low temperatures and flammability, which are common with conventional electrolytes.
- Ionic Conductivity: Mixtures with lithium bis(trifluoromethylsulfonyl)imide ([Li][TFSI]) exhibit ionic conductivities ranging from 0.4 S/m to 0.1 S/m at room temperature, making them highly efficient for lithium-ion battery applications.
- Amorphous Behavior: The addition of lithium salts increased the amorphous nature of the mixtures, which improves ionic mobility and storage capabilities.
These findings place [C3C1Pyrr][TFSI] as a promising candidate for enhancing the safety and performance of lithium-ion batteries, with potential applications in other areas of green technology.
Real-World Applications
- Energy Storage Systems: [C3C1Pyrr][TFSI] is pivotal in creating safer, longer-lasting lithium-ion batteries that can power everything from electric vehicles to renewable energy grids.
- Industrial Processes: Its ability to dissolve a wide range of organic, inorganic, and polymeric substances makes it invaluable in specialized chemical industries.
- Sustainable Technologies: The ionic liquid contributes to greener manufacturing processes by replacing volatile organic solvents, thereby reducing environmental impact.
Why Choose RoCo for [C3C1Pyrr][TFSI]?
At RoCo, we’re committed to providing high-quality materials that meet the demands of advanced scientific and industrial applications. Our [C3C1Pyrr][TFSI] stands out for its high purity and consistent performance, backed by rigorous quality control and cutting-edge research.
Join the Innovation Revolution
The future of energy and material science is here. Whether you’re developing safer batteries, exploring sustainable solvents, or driving advancements in green chemistry, 1-Methyl-1-propylpyrrolidinium bis(trifluoromethylsulfonyl)imide is your key to success. Explore its possibilities and redefine the boundaries of innovation with RoCo.