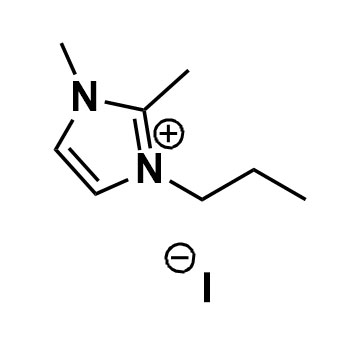
1,2-Dimethyl-3-propylimidazolium iodide, >98%
$75.64 – $3,254.96
Product Code: IL-0049-HPCAS NO: 218151-78-1
- Chemical Formula: C8H15IN2
- Synonyms: DMPIIm, DMPII, 1-Propyl-2,3-dimethyl-imidazolium iodide
- SPECIFIC GRAVITY: NA
- SUM Formula: C8H15IN2
- Molecular Weight: NA
- Melting Point: 94°C
- Density: NA
- ECW: NA
- HMIS KEY: NA
- TSCA: NA
- Viscosity: NA
1,2-Dimethyl-3-propyl imidazolium Iodide (218151-78-1):
1,2-Dimethyl-3-propyl imidazolium iodide (DMPII) is an organic compound with the molecular formula C8H15IN2 and the CAS number 218151-78-1. It is also known as 1,2-dimethyl-3-n-propyl imidazolium iodide or 1-propyl-2,3-dimethyl imidazolium iodide. DMPII is an ionic liquid that can act as a multiple-functional redox mediator (RM) for Li-O2 batteries. It can promote the oxygen reduction reaction (ORR) by facilitating the formation of LiO2, reduce the charge potential by oxidizing LiO2, and protect the Li anode from redox shuttling by in situ generating a “self-defense” solid electrolyte interphase (SEI) layer. DMPII has a melting point of 94 °C. Besides Li-O2 batteries, DMPII can also be used as a component of ionic liquids for other applications, such as organic synthesis, catalysis, separation, and electrochemistry. DMPII can be synthesized by reacting 1-methylimidazole with 1-bromopropane and methyl iodide in a two-step process.
Key Applications
Li-O2 batteries: A study by Li et al. (2021) showed that DMPII can improve the performance and stability of Li-O2 batteries by acting as a multiple-functional redox mediator. They demonstrated that a cell with DMPII can achieve stable cyclability with a low terminal charge potential of ~3.6 V till the cell death, a considerable rate performance, and good reversibility associated with Li2O2 formation and degradation.
Organic synthesis: A study by Zhang et al. (2010) showed that DMPII can act as a solvent or a catalyst for various organic reactions, such as alkylation, acylation, condensation, and oxidation. They reported that DMPII can enhance the reaction rate and yield by improving the solubility and reactivity of the reactants.
Catalysis: A study by Zhang et al. (2006) showed that DMPII can act as a support or a modifier for metal or metal oxide catalysts, enhancing their activity and selectivity for reactions such as hydrogenation, oxidation, and CO2 conversion. They reported that DMPII can modify the catalysts’ electronic structure and surface properties by forming coordination bonds or charge transfer complexes.
Separation: A study by Qiao et al. (2017) showed that DMPII can act as a solvent or an extractant for separating mixtures of organic or inorganic compounds, such as alcohols, esters, acids, and metals. They reported that DMPII can achieve high separation efficiency and selectivity by exploiting its polarity, hydrogen bonding ability, and coordination capability.
Electrochemistry: A study by Li et al (2020) and Sutton et al (2022) shows that DMPII can act as an electrolyte or an additive for electrochemical devices or processes, such as supercapacitors, fuel cells, and electroplating. They also report that DMPII can improve the electrolyte’s conductivity, stability, and compatibility by forming ion pairs or complexes with the electrodes or additives.
Please contact us if you want to learn more or need assistance with your order.