Ionic Liquids in Industrial Applications
Research in the field of ionic liquids (ILs) has increased since their initial discovery in 1914 by Paul Walden. Ionic liquids, salts in liquid state, are frequently described as being liquid below an arbitrary value of 100°C. However, one should not use this constraint for a substance to be considered an IL. We think of any organic salt which melts without decomposition as an ionic liquid at that temperature. The organic nature of ILs is attributed to the bulky and unsymmetrical structure of the constituent ions, which can be paired together in different combinations to target specific properties such as electrical, chemical, and thermal. 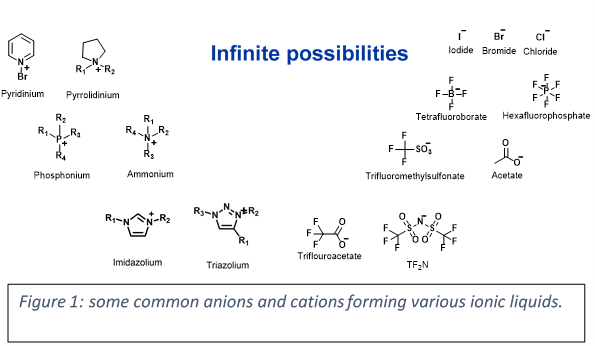
Some examples of common cations and anions are shown above with together with their preferred names.
ILs have been investigated in an extensive range of applications including solvents, catalysts, lubricants, and in a number of electrochemical applications such as batteries, super capacitors etc.
Due to the wide range of tunable properties, ILs are deemed as material of the future. ILs have been slowly but surely finding their way in several industrial processes which heavily rely on toxic, flammable and highly volatile systems. The low vapor pressure of ILs has been of particular interest due to their ability to lower the atmospheric pollution. However, not all ILs are environmentally friendly. Depending on the chemical structure, some ILs can be extremely toxic to humans and the environment. IL toxicity, particularly toward aquatic organisms, has been identified as an emerging problem as the use of ILs increases.
ILs are thermal stability and due to their ionic nature they are generally non-flammable. Their low vapor pressure at ambient temperature has also garnered quite a bit of interest in decreasing volatile organic content (VOC). These properties have been quite useful in increasing the safety of high temperature applications. One of the areas which ILs have had a major impact is in energy storage resulting in improved performance and safety.
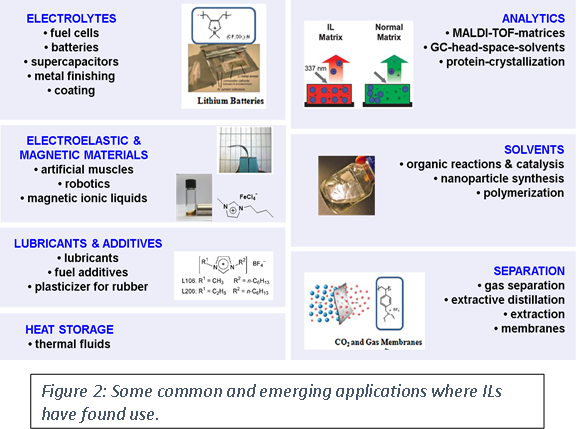
The unique properties of ILs have opened up new applications specifically in ultra-low vacuum and in a number of outer space applications. Additionally, as ILs consist largely of ions, they also have higher thermal and electrical conductivities in comparison to common solvents, and much larger electrochemical windows. This has meant that they have found quite a few uses, see figure 2.
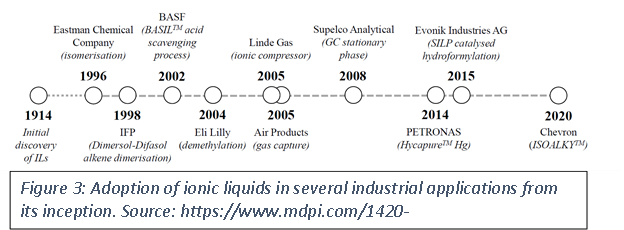 Adoption of ILs in a number of industrial applications has bloomed since their discovery (Figure 3). Today, ionic liquids are found in cell phones, tablets and in various displays. ILs are now found in a number of commercial plastics specifically high end applications such as an anti-static agents, to improve dispersibility etc. Figure 3 shows some industrial application of ionic liquids by various large organizations.
Adoption of ILs in a number of industrial applications has bloomed since their discovery (Figure 3). Today, ionic liquids are found in cell phones, tablets and in various displays. ILs are now found in a number of commercial plastics specifically high end applications such as an anti-static agents, to improve dispersibility etc. Figure 3 shows some industrial application of ionic liquids by various large organizations.
The possibilities for IL application seem endless. It’s important to understand the structure property relationships of ILs to maximize their performance in a specific application while minimizing their environmental impact. Are you looking for an ideal partner to help you rapidly advance material initiatives? Learn more how can we help you with material application by using ionic liquids as additives in your application. Contact RoCo Global today to learn more about our Research & Development Services and how we can help you meet, and exceed, your goals.
