Metal Free Battery: Ionic Liquids
It is estimated that there are about 15 billion mobile devices today. The number of devices will increase to 50 billion by 2030. As we become more connected, use more electric vehicles, and integrate renewable energy into our daily lives, demand will increase for energy storage. When it comes to portable energy, lithium-ion batteries (LIB) are the king. LIBs have found their way in all sorts of new devices with a net improvement in our lives. In fact, LIBs have made widespread adoption possible for smartphones. In the past 25 years, LIB improvements have focused on continuously increasing their specific energy (Wh kg−1) and energy density (Wh L−1). The use of LIBs in automotive is now the hottest growth area.

Figure 1: Future Battery Performance Parameters
Moving away from fossil energy absolutely necessary. However, we need to be cognizant of environmental hazards with battery technologies, especially LIBs. We certainly don’t want to develop new technology with dire long-term environmental consequences. LIBs are not hazard-free, and the public notion that they are environmentally friendly is wrong. It is important to note that 99.5% of lead-acid batteries are recycled. However, no real recycling effort exists in batteries such as LIBs for consumer electronics, and the exact combination and number of chemicals inside a battery vary drastically. Many batteries include metals like cadmium, nickel, cobalt, copper, iron, and lithium. The newer generation of battery electrolytes also contains fluorinated electrolytes, which can be toxic if not disposed of properly. When batteries are thrown into household trash, they end up in landfills, ultimately leaching chemicals into the soil and making their way into our water and food supply. If the batteries are burned, they can release highly toxic and hazardous compounds.
Depending on the application, battery types change. For example, power tools vs. portable electronics have different needs in terms of energy output and sustained performance. Battery manufacturers are aware of the energy needs of the specific device. However, as the number of battery power devices grows, it is now clear that we need to think about additional key parameters. These include 1) the CO2 footprint of the battery in its production and use. 2) Reducing the use of metals such as Cd, Li, Co, or Ni 3) Using more sustainable or “green” materials such as sodium and potassium 4) the design of batteries needs to be such that they are 100% recyclable (lead-acid batteries are almost 99% recycled). 5) additional incentives such as tax credits towards green batteries.
Indeed, there are emerging technologies that utilize much lower toxicity and abundant materials. Specifically, the dual-ion battery (DIB) is safer and much easier on the environment (compared to mining cobalt and lithium). DIB uses positive and negatively charged ions that are active in energy storage, as shown in Figure 2.
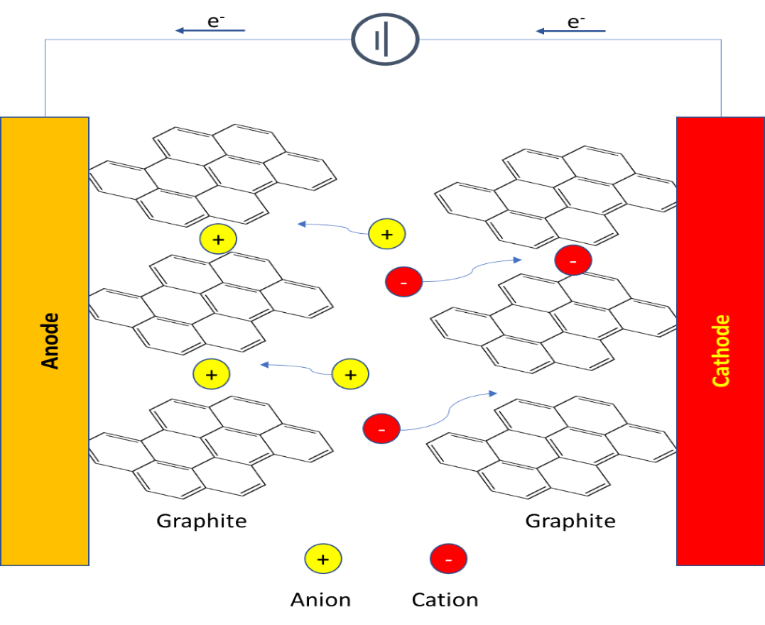
Figure 2: Schematic illustration of the dual-graphite battery (DGB) system
during the charging process, using ionic liquids as the electrolyte.
DIBs have strong potential for high energy density and low-cost, safe materials, but problems with stability and electrolyte performance at higher voltages have been challenging. DIBs can be charged and discharged at much larger currents and are very suitable for high-power applications such as moving large, heavy objects at high speed.
Ionic liquids hold the potential to overcome the high voltage problems associated with DIBs and can also be used in the development of metal-free devices. The high working voltage of DIBs makes ionic liquids, especially feasible as electrolytes with high working voltages. Recently, Wang et al. reported a novel dual-graphite battery system using pure 1-Butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide ionic liquid. The DIB developed in this paper has no metallic elements and was investigated in great detail about its performance. This work opens up new possibilities for making batteries without any metals and the possibility for easy recycling and reuse.
Are you looking for an ideal partner to help you rapidly advance various technologies? Perhaps you want to design and test custom gas separation membranes for your application, an improved battery electrolyte, or an additive to reduce viscosity?
Contact RoCo® today to learn more about our Research & Development Services and how we can help you meet, and exceed, your goals.
