Rethinking Power: The Rise of Sodium(Na-ion) and Potassium (PIB) Batteries and the Need for U.S. Engagement
Blog By Hunaid Nulwala, Ph.D
Lithium-ion batteries have long been the standard for energy storage, powering a vast array of devices from smartphones to electric vehicles and the electric grid. However, lithium’s limited availability and exorbitant cost are causing significant concerns in the supply chain. Fortunately, promising alternatives such as Sodium-ion (Na-ion) and Potassium-ion (PIB) batteries can effectively address lithium shortages and be a dependable option for large-scale energy storage.
Sodium-Ion Batteries: A Bright Prospect
Na-ion batteries are a more affordable and widely available alternative to lithium-ion batteries. Due to their similar electrochemistry, they are becoming an increasingly popular choice. The abundance of sodium makes these batteries an even better option. Over the past ten years, Na-ion batteries have made significant progress and can replace lithium-ion batteries in specific applications, such as stationary storage.
Chinese companies and automakers have made impressive progress in developing sodium-ion battery technology. JAC Motors, a Chinese automaker, recently revealed a vehicle powered by a 25-kilowatt-hour sodium-ion battery produced by HiNa Battery. The car can travel up to 250 kilometers (155 miles) on a single charge. CATL, China’s biggest EV (Electric Vehicle) battery maker, has also developed a sodium-ion battery for use in a Chery vehicle.
Scientists are working on developing anode-free sodium (Na) metal batteries that are both cost-effective and environmentally friendly. The goal is to create energy storage systems comparable to lithium-ion batteries in terms of energy density and safety while reducing environmental impact. With their low cost, high safety, broad temperature range, and sustainability, these Na metal batteries have the potential to become a viable commercial option.
Potassium-Ion Batteries: Full of Potential
Potassium-ion batteries are noteworthy for their abundant raw materials, high energy density, fast ion transport in the electrolyte, and lower cost. An advantage of potassium-ion batteries is that they can use more inexpensive and abundant materials such as potassium, iron, and aluminum instead of expensive ones like lithium, cobalt, and copper. Additionally, PIBs have a lower fire risk than lithium batteries, making them a safer option.
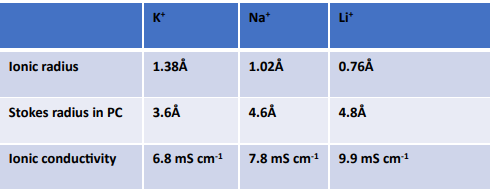
To give you a visual, here is a handy comparison of ionic and conductivity among potassium, sodium, and lithium:
Table 1: Difference in ionic radius, stokes radius, and ionic conductivity. 1
Adopting sodium-ion and potassium-ion batteries could significantly lessen the demand for lithium, cobalt, and nickel, freeing up these resources for next-generation high-energy-density lithium-ion batteries. Potassium (K+) is larger than sodium (Na+) and lithium (Li+); therefore, it has a lower charge density. However, let’s consider its transport behavior, as shown in Table 1. K+ has the lowest energy of solvation/desolvation of the three, as reported by Okoshi.2 This lower energy enables a fast desolvation process at the electrode-electrolyte interface with beneficial effects on rate capability. In LIBs, Abe et al. demonstrated that one of the critical rate-limiting processes of Li intercalation into the graphite electrode is the desolation process, suggesting that potassium ion batteries may be more suitable for high-power applications.3 Also, K+ in solution typically has a smaller stokes radius due to weaker interactions with solvent molecules which may facilitate higher ion transport.
The United States must prioritize improving its sodium and potassium-ion battery technology to keep up with China’s advancements in non-lithium battery technologies. There are numerous sodium and potassium salts and ionic liquids that show great promise for the development of these batteries. With advances in battery chemistry, cell engineering, and system integration, sodium and potassium batteries could become widely used within the next decade. These batteries could drastically transform the energy landscape, from portable electronics to electric vehicles and grid energy storage. Promising materials and ionic liquids such as Potassium trifluoromethanesulfonamide, sodium trifluoromethanesulfonamide, Sodium tricyanomethanide, Sodium dicyanamide, N-methyl-N-propylpyrrolidinium bis(fluorosulfonyl)imide (Pyr13FSI), 1-Ethyl-3-methylimidazolium bis(fluorosulfonyl)imide (EMim FSI), and 1-Butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide (PYR14 TFSI) can be used for developing such batteries.
Sodium and potassium batteries have the potential to be widely used in various applications such as portable electronics, electric vehicles, and grid energy storage. With the proper support, including advancements in battery chemistry, cell engineering, and system integration, these batteries could be put into operation within the next ten years. This is expected to impact our society’s energy landscape significantly.
References:
(1) Hosaka, T.; Kubota, K.; Hameed, A. S.; Komaba, S. Research Development on K-Ion Batteries. Chem Rev 2020, 120 (14), 6358–6466. https://doi.org/10.1021/acs.chemrev.9b00463
(2) Okoshi, M.; Yamada, Y.; Komaba, S.; Yamada, A.; Nakai, H. Theoretical Analysis of Interactions between Potassium Ions and Organic Electrolyte Solvents: A Comparison with Lithium, Sodium, and Magnesium Ions. J Electrochem Soc 2017, 164 (2), A54–A60. https://doi.org/10.1149/2.0211702jes
(3) Abe, T.; Fukuda, H.; Iriyama, Y.; Ogumi, Z. Solvated Li-Ion Transfer at Interface Between Graphite and Electrolyte. J Electrochem Soc 2004, 151 (8), A1120. https://doi.org/10.1149/1.1763141