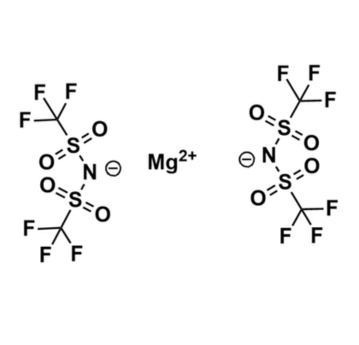
Magnesium bis(trifluoromethylsulfonyl)imide, >99%
Product Code: KI-0057-HPCAS NO:133395-16-1
- Chemical Formula: C4F12MgN2O8S4
- Synonyms: Mg (BTA)2
Price upon request.
**This product will incur a $97.00 HazMat fee when the order is placed.**
Please email us at info@roco.global to place an order
Category: Metal Salts
SUM Formula: C4F12MgN2O8S4
Molecular Weight: 584.60
Purity: >99%
- SUM Formula: C4F12MgN2O8S4
- Molecular Weight: 584.60
Magnesium bis(trifluoromethylsulfonyl)imide, CAS: 133395-16-1
Key Applications:
Advanced Electrolyte Systems
- Serves as a high‑purity magnesium salt for next‑generation rechargeable Mg‑ion batteries, enabling non‑nucleophilic, chloride‑free electrolyte formulations.
- Supports high oxidative stability and wide electrochemical windows in ether‑based, sulfone‑based, and ionic‑liquid‑based electrolytes.
- Used in research on multivalent metal batteries where reduced dendrite formation and improved cycling efficiency are required.
- Facilitates development of hybrid electrolytes combining Mg(TFSI)_2 with polymer matrices (PEO, PVDF, polycarbonates) for solid‑state or gel systems.
Ionic Liquid and Solvate Electrolytes
- Dissolves readily in fluorinated and aprotic solvents to form stable solvate structures for high‑conductivity electrolyte blends.
- Employed in Mg‑compatible ionic liquid systems based on TFSI anions for enhanced thermal stability and suppressed parasitic reactions.
- Useful in tailoring viscosity, ion mobility, and interfacial behavior in designer electrolyte formulations.
Electrodeposition and Surface Engineering
- Enables controlled magnesium electrodeposition in non‑aqueous media for thin‑film formation, protective coatings, and surface modification studies.
- Investigated for corrosion‑resistant Mg coatings and interfacial engineering in metal–electrolyte systems.
Catalysis and Lewis Acid Chemistry
- Functions as a moisture‑stable, non‑coordinating magnesium source for Lewis acid catalysis in organic synthesis.
- Applied in polymerization, activation of electrophiles, and transformations requiring weakly coordinating anions.
Materials Science and Coordination Chemistry
- Used to prepare Mg‑based coordination complexes with TFSI counterions for structural, spectroscopic, and conductivity studies.
- Supports development of Mg‑containing functional materials, including ionogels, composite electrolytes, and high‑temperature materials.
Analytical and Reference Uses
- Serves as a reference salt for studying Mg^{2+} solvation, transport behavior, and ion pairing in highly fluorinated anion systems.
- Utilized in benchmarking electrolyte stability, decomposition pathways, and interphase formation mechanisms.
Please contact us if you want to learn more or need assistance with your order.
- Yu, Z., Juran, T. R., Liu, X., Han, K. S., Wang, H., Mueller, K. T., Ma, L., Xu, K., Li, T., Curtiss, L. A., Cheng, L. 2023. Solvation structure and dynamics of Mg(TFSI)_2 aqueous electrolyte. Journal Article.
- Baskin, A., Prendergast, D. 2016. Exploration of the detailed conditions for reductive stability of Mg(TFSI)_2 in diglyme: implications for multivalent electrolytes. J. Phys. Chem. C 120, 3583–3594.
Related products
-
Zinc bis(trifluoromethylsulfonyl)imide, >98%
$120.27 – $1,195.17Price range: $120.27 through $1,195.17Product Code: KI-0062-HP -
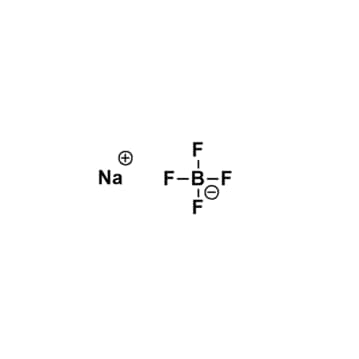
Sodium tetrafluoroborate, >98%
$56.53 – $215.72Price range: $56.53 through $215.72Product Code: KI-0025-HP -
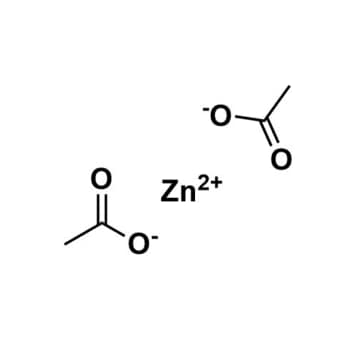
Zinc acetate, >99%
$78.50 – $754.60Price range: $78.50 through $754.60Product Code: ROCO-003zn -
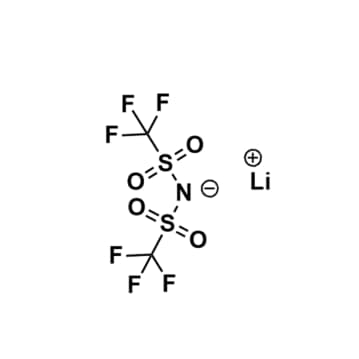
Lithium bis(trifluoromethylsulfonyl)imide, >99%
$117.42 – $2,090.56Price range: $117.42 through $2,090.56Product Code: KI-0001-HP