Summary and Opinion by Hunaid Nulwala Ph.D.
Introduction: Lithium-sulfur (Li-S) batteries have emerged as promising candidates for next-generation energy storage systems due to their high specific energy capacity and density. However, challenges such as sulfur’s insulating nature and the large volume change of S/Li2S during discharge/charge processes hinder the full utilization of sulfur in Li-S batteries.
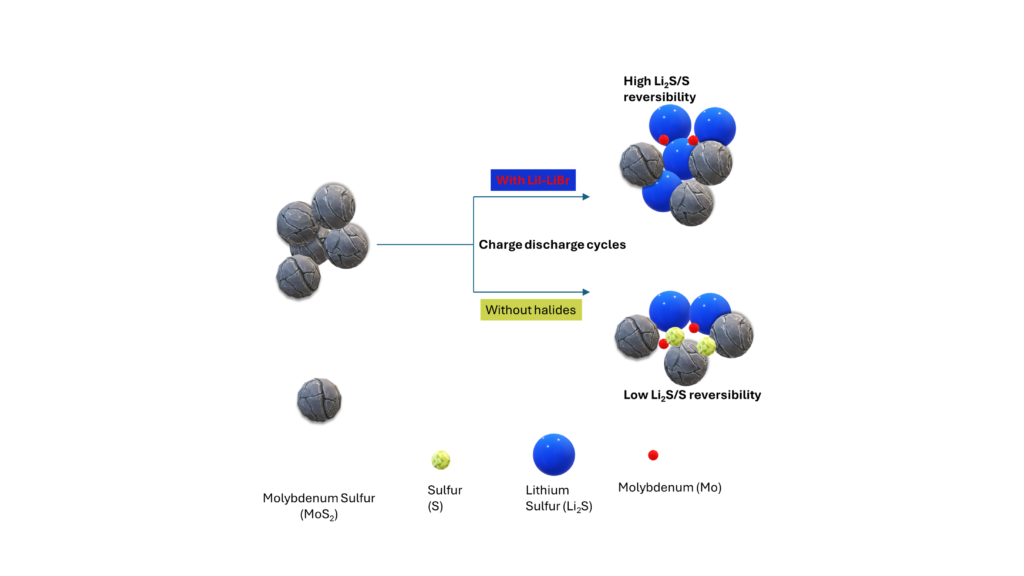
Figure 1: The addition of halides in the MOS2 results in improved Lithium Sulfur battery performance.
To overcome these limitations, researchers have been exploring various strategies to improve the Li2S/S reaction and enhance the overall performance of Li-S batteries. One such approach is the incorporation of bromine (Br) and iodine (I) into Li2S with carbon black. This work was quite interesting because halides are known to be found in many ionic liquids. It makes me think that not all halide is likely detrimental, and certainly, there is some synergy. However, the control of halide content is important. The work performed by Wang et al, 1 caught my attention. After ball-milling carbon black and MoS2 with LiI-LiBr, the particle size of MoS2 with LiI-LiBr is reduced. Overall, they observed enhanced electronic conductivity and improved MoS2 reversibility and kinetics.
My thoughts stem from varied research results found in the battery space by various research groups. I firmly believe that halides play a significant role in all energy storage devices that use ionic liquids. I have come to believe that the incorporation of various halides as additives in ionic liquids can significantly improve the performance of lithium-ion batteries.
A note of caution: Significant side reactions can occur. One must think about the whole system, not just the components.
Potential implication of a small amount of halide in a Li-S battery:
- Complex Defect Formation: When Br/I is introduced into Li2S, complex defects are formed, including Li vacancies, along with Br-on-S or I-on-S substitutions. These arise due to valence inconsistencies between Br/I and S. The formation of these defects plays a crucial role in enhancing the Li2S/S reaction, this can enhance kinetics.
- Reduced Li-S Bond Strength: The complex defects formed by Br/I incorporation reduce the bonding strength of the Li-S bond. In this paper, Crystal Orbital Hamilton Population (COHP) analysis reveals that the bonding state of the Li-S bond around complex defects decreases, leading to a weakened Li-S bond. This weakened bond facilitates the delithiation of Li2S during the discharge process.
- Enhanced Li-Ion Conductivity: Incorporating Br/I into Li2S significantly improves ionic conductivity. Li2S typically exhibits low ionic conductivity (10^-13 S cm^-1). However, introducing LiI alone increases the ionic conductivity to 6.4 × 10^-8 S cm^-1 in the Li2S@LiI composite. Furthermore, incorporating the LiI-LiBr compound further enhances the ionic conductivity, resulting in 7 orders of magnitude increase (1.08 × 10^-6 S cm^-1) compared to Li2S. One can think about the size of halides. But there is not enough information to guess or provide insight.
- Unknown side reactions: Halides are reactive, and if not properly handled, they can result in unwanted side reactions that harm the device and can be a major safety concern. One has to use halides as an additive in a controlled manner.
The authors in this publication also performed Density Functional Theory (DFT) calculations to support the experimental findings. They show that LiI-LiBr incorporation reduces the formation energy of complex defects in Li2S. The lower formation energy indicates the stability and effectiveness of these complex defects in improving the Li2S/S reaction.
Conclusion: Br/I incorporation in Li2S is a promising strategy for enhancing the Li2S/S reaction in lithium-sulfur batteries. The formation of complex defects, the weakened Li-S bond strength, and the improved Li-ion conductivity contribute to the overall improvement in the electrochemical performance of Li-S batteries. These advancements bring us closer to realizing the full potential of Li-S batteries as a high-capacity and energy-dense energy storage solution.
Numerous strategies can be incorporated to improve lithium-based batteries. Indeed, controlled impurities of halides other than fluoride can significantly improve battery performance.
At RoCo, we are developing carbon specifically enhanced with ionic liquids to improve battery performance. These materials will be added to our store to be purchased shortly for improving your battery research.
Reference:
(1) Wan, H.; Zhang, B.; Liu, S.; Zhang, J.; Yao, X.; Wang, C. Understanding LiI-LiBr Catalyst Activity for Solid State Li2S/S Reactions in an All-Solid-State Lithium Battery. Nano Lett 2021, 21 (19), 8488–8494. https://doi.org/10.1021/acs.nanolett.1c03415.