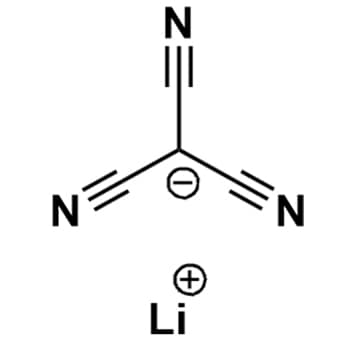
Lithium tricyanomethanide, >99%
$413.22 – $4,848.63Price range: $413.22 through $4,848.63
Product Code: KI-0056-HPCAS NO: 210043-80-4
- Chemical Formula: C4N3Li
- Synonyms: Li+ -C(CN)3
**This product will incur a $97.00 HazMat fee when the order is placed.**
Category: Metal Salts
SUM Formula: C4N3Li
Molecular Weight: 97.00
Purity: >99%
- SUM Formula: C4N3Li
- Molecular Weight: 97.00
Lithium tricyanomethanide, CAS: 210043-80-4
Key Applications
Advanced Electrolyte & Energy‑Storage Research
- Serves as a high‑stability anionic component for next‑generation lithium battery electrolytes, where the tricyanomethanide anion offers strong charge delocalization and low nucleophilicity.
- Investigated as a lithium‑salt dopant in ionic liquid matrices to improve ionic conductivity and broaden electrochemical windows.
- Useful in solid‑state electrolyte design, particularly in polymer–salt complexes requiring highly dissociated lithium sources.
Organometallic & Coordination Chemistry
- Acts as a versatile ligand precursor, enabling formation of metal–tricyanomethanide complexes with tunable electronic properties.
- Supports synthesis of electron‑poor coordination frameworks, including materials for redox‑active assemblies and molecular electronics.
- Employed in anion‑exchange reactions to introduce the tricyanomethanide motif into metal centers or cationic organic scaffolds.
Organic Synthesis & Functional Materials
- Provides a clean, high‑purity source of the tricyanomethanide anion for nucleophilic substitution, anion metathesis, and construction of highly conjugated, electron‑withdrawing functional groups.
- Used in the preparation of push–pull chromophores, donor–acceptor polymers, and molecular conductors where strong electron‑accepting behavior is required.
- Supports synthesis of ionic organic semiconductors and charge‑transfer materials.
Catalysis & Reaction‑Medium Engineering
- Functions as a weakly coordinating anion in catalytic systems, stabilizing reactive cationic intermediates without interfering with catalytic turnover.
- Useful in ionic‑liquid‑based catalysis, where lithium salts modulate viscosity, polarity, and ion‑pairing behavior.
- Enables development of low‑nucleophilicity reaction media for sensitive transformations.
Materials Science & Specialty Applications
- Applied in the design of electroactive polymers, metal–organic frameworks, and porous materials requiring highly delocalized anions.
- Supports research into high‑dielectric materials and tunable ion‑pair systems for advanced electronics.
- Used in spectroscopic and mechanistic studies where the tricyanomethanide anion provides a distinct, well‑resolved signature.
Please contact us if you want to learn more or need assistance with your order.
-
Tong, B., Song, Z., Wu, H., Wang, X., Feng, W., Zhou, Z., & Zhang, H. (2022). Ion transport and structural design of lithium-ion conductive solid polymer electrolytes: a perspective. Materials Futures, 1(4), 042103.
-
Santiago, A., Judez, X., Castillo, J., Garbayo, I., Sáenz de Buruaga, A., Qiao, L., Baraldi, G., Antonio Coca-Clemente, J., Armand, M., Li, C., & Zhang, H. (2020). Improvement of Lithium Metal Polymer Batteries through a Small Dose of Fluorinated Salt. The Journal of Physical Chemistry Letters, 11(15), 6133–6138.
Related products
-
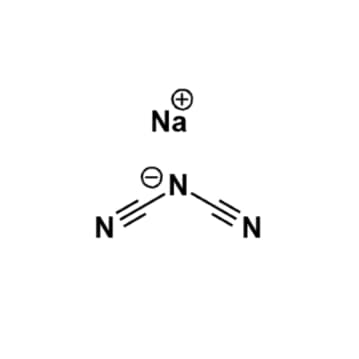
Sodium dicyanamide, >97%
$168.35 – $1,450.81Price range: $168.35 through $1,450.81Product Code: KI-0015-SG -
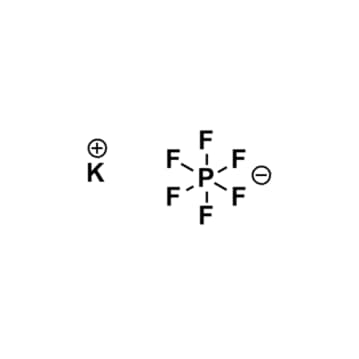
Potassium hexafluorophosphate, >99%
$120.27 – $1,058.58Price range: $120.27 through $1,058.58Product Code: KI-0031-HP -
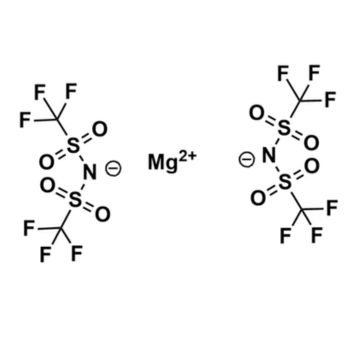
Magnesium bis(trifluoromethylsulfonyl)imide, >99%
Product Code: KI-0057-HP -
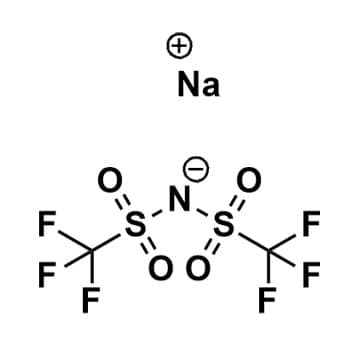
Sodium Bis(trifluoromethanesulfonyl)imide, 97%
$65.00 – $365.00Price range: $65.00 through $365.00Product Code: ROCO-012