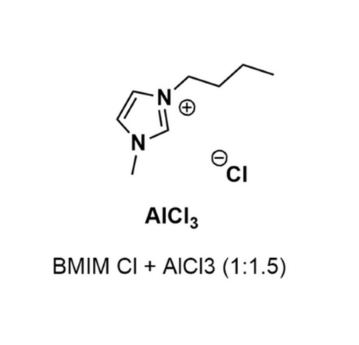
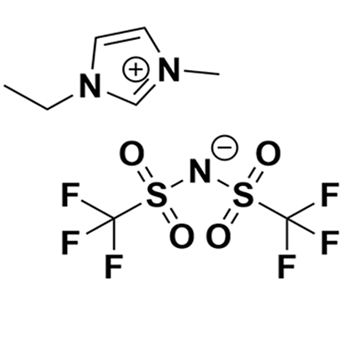
1-Butyl-3-methylimidazolium chloride and Aluminum chloride (1:1.5)
$75.64 – $503.68
Product Code: EP-0002-HPCAS NO: 80432-09-3
- Chemical Formula: C₈H₁₅N₂]Cl·1.5AlCl₃
- Synonyms: BMIM Cl/AlCl3 (1:1,5), BMIM AlCl4
- Electrolyte for aluminum deposition
*This product will incur a $95 HazMat fee when the order is placed.*
- SUM Formula: C₈H₁₅N₂]Cl·1.5AlCl₃
- Molecular Weight: ~374.68
- Density: 1.3-1.4g/cm³
1-Butyl-3-methylimidazolium chloride and Aluminum chloride (1:1.5) (80432-09-3)
Key Applications:
- Catalysis: Accelerate organic transformations with this ionic liquid’s potent Lewis acidity, ideal for alkylation, acylation, and oligomerization reactions.
- Solvent Systems: Leverage its unique solvation properties for homogeneous catalysis, extraction processes, or as a green alternative to volatile organic solvents.
- Electrochemistry: Utilize its wide electrochemical window for advanced battery research, electrodeposition, or electrochemical sensing.
- Material Synthesis: Facilitate the preparation of novel polymers, nanomaterials, or composites with its versatile reactivity.
Handling and Storage:
- Moisture Sensitivity: This product is highly hygroscopic and reacts with water, forming HCl. Handle under inert atmosphere (e.g., nitrogen or argon) using glovebox or Schlenk techniques.
- Storage: Store in a tightly sealed container in a cool, dry place away from moisture and air. Recommended storage temperature: 2–8 °C.
- Safety: Wear appropriate PPE (gloves, goggles, lab coat) and work in a well-ventilated area or fume hood. Consult SDS for detailed safety information.
Why Choose [BMIM]Cl/AlCl₃ (1:1.5)?
Our chloroaluminate ionic liquid is meticulously crafted to meet the needs of researchers and industrial chemists pushing the boundaries of catalysis, electrochemistry, and green chemistry. Its acidic composition, low melting point, and robust stability make it a game-changer for innovative applications. Unlock new possibilities in your lab with this dynamic ionic liquid!